Jump to Section
What is iLink Corneal Cross Linking?
iLink corneal cross linking (CXL) is the only FDA-approved cross-linking treatment for progressive keratoconus. It is a minimally invasive outpatient procedure which combines the use of ultraviolet light and specially formulated eye drops to stiffen and strengthen the cornea that has been weakened by disease or refractive surgery by creating connections between collagen fibers found within this area of the eye. It’s perfect for those who aren’t able or willing to have corneal cross linking surgery.
Cross-linking (also known as corneal collagen cross linking or corneal crosslinking) is considered the standard of care around the world for progressive keratoconus and corneal ectasia following refractive surgery and can prevent the need for a corneal transplant.
Is the Corneal Cross Linking Procedure Right for Me?
Patients who have been diagnosed with progressive keratoconus or corneal ectasia following refractive surgery should ask their doctor about iLink corneal cross-linking. The best candidates for corneal cross linking have some damage or degeneration to their corneas and are experiencing corneal haze, but the cornea is not too irregular or misshapen, and significant vision loss has not yet taken place. The procedure is best for those who are in the early stages of a disease.
Our practice is proud to offer patients the first and only FDA-approved therapeutic solution for the treatment of progressive keratoconus. Now, patients who once had little to no therapeutic option to treat keratoconus have the opportunity to slow or halt the progression of this sight-threatening disease.
For more information about the iLink procedure for the treatment of progressive keratoconus and corneal ectasia following refractive surgery, visit www.glaukos-iLink.com
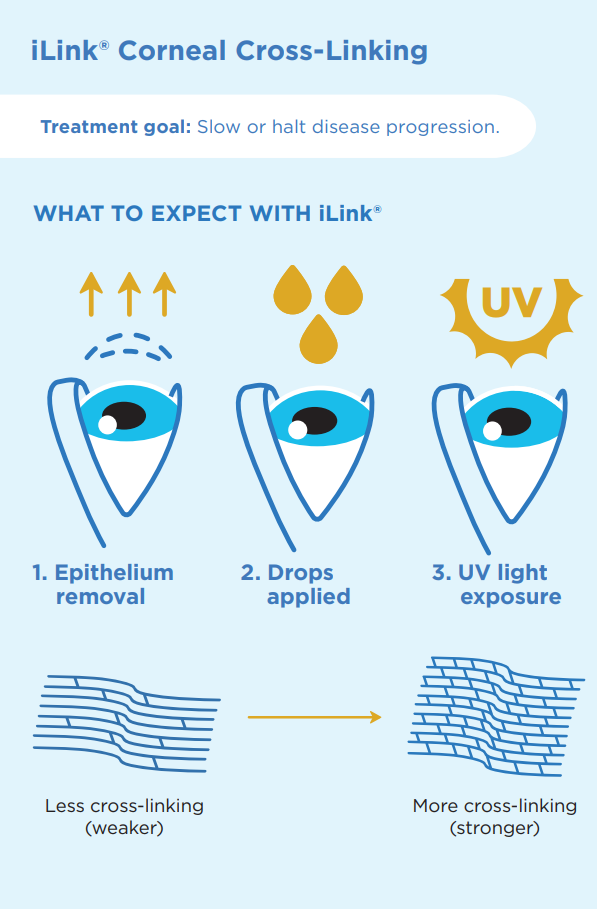
How is iLink Corneal Cross Linking Performed?
Before your corneal cross linking procedure is performed, your ophthalmologist will complete an eye exam and measure the thickness of your cornea. This ensures that you are a good candidate for the procedure. They will also create a detailed map of the shape of your cornea; this is known as corneal topography.
The corneal cross linking procedure can be completed in about an hour. Your ophthalmologist will first remove the epithelium, which is the outer layer of the cornea. Then, specially formulated pharmaceutical-strength riboflavin (vitamin B2) drops called Photrexa® (riboflavin 5’-phosphate ophthalmic solution) and Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) are applied to the eye and allowed to absorb into the cornea to help enable the cross-linking reaction. Next, a UV light from a machine called the KXL system is applied for about 30 minutes before applying bandage contact lenses to the eye. The iLink procedure stiffens and strengthens the cornea to slow or halt the progression of keratoconus and preserve vision. The procedure is typically not painful, as numbing drops are applied to the eyes and patients are given mild sedation prior to the cross linking procedure.
Recovery After Corneal Cross Linking
After corneal cross linking, you may experience some mild discomfort which most patients describe as a dry, burning, or gritty sensation in the eye. Pain should not be severe, and you should contact your ophthalmologist if you do experience severe discomfort. Sensitivity to light is also normal during the healing process after corneal cross linking.
Is iLink Covered by Insurance?
The medical necessity of iLink has become widely recognized. As a result, commercial insurance coverage for the procedure is now over 95% in the United States.
Schedule an Appointment
Corneal cross linking is an advanced procedure that few ophthalmologists offer. To learn more about the procedure, book an appointment online or try giving us a call at 617-202-2020.
Our Cornea Services, including Cornea cross linking, are available at all our locations: Brookline, Milford, Medford, Lawrence, Wellesley, Burlington, and Andover.